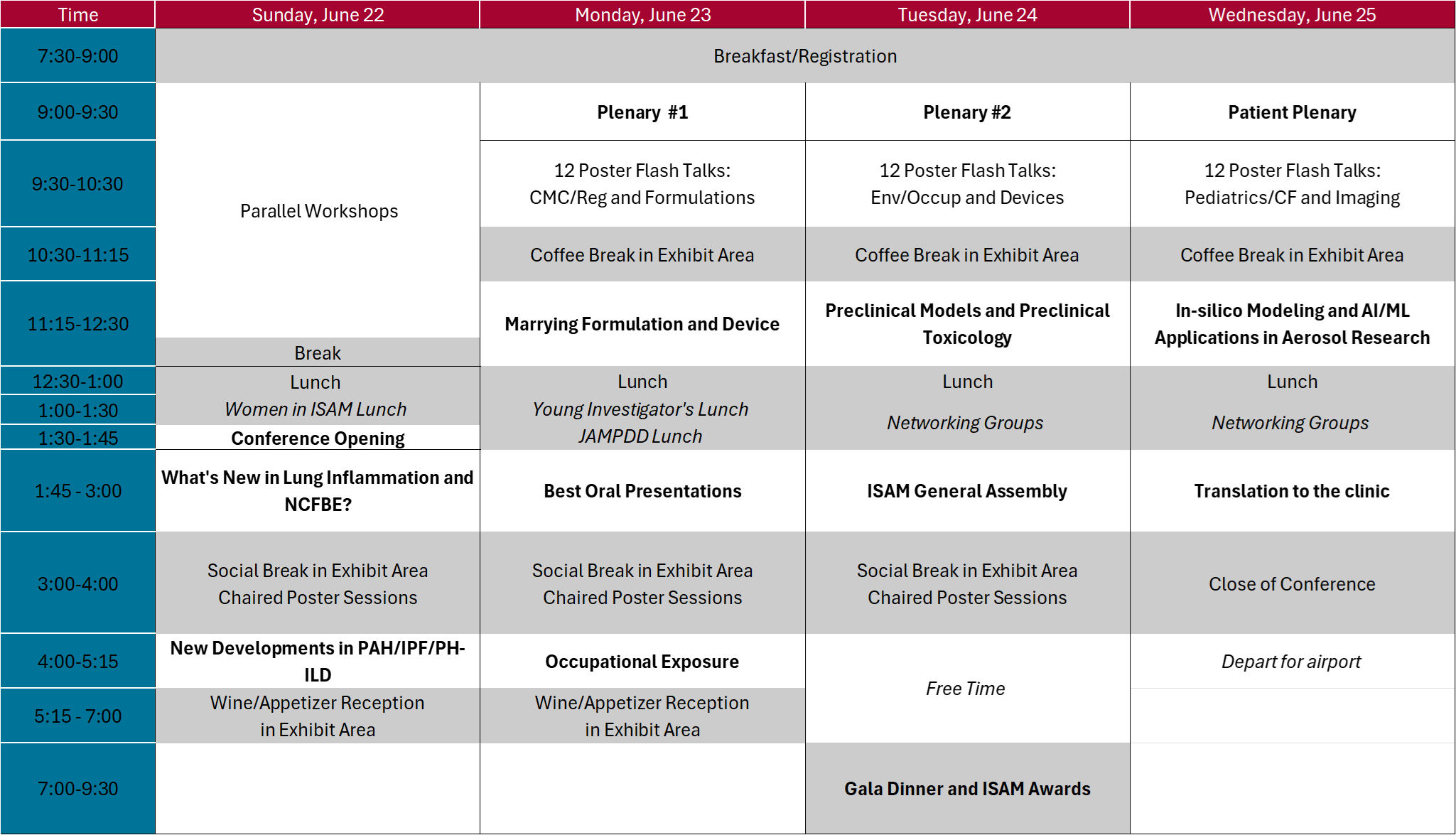
ISAM Conference Workshop Overview
Sunday 22 June 2025
8:00 am Registration Opening
9:00 - 12:00 Comprehensive Approaches to Inhalation Safety and Toxicology: From Pharmaceuticals to Environmental Aerosols
This workshop aims to provide an overview on current models and methodologies to assess safety assessment of inhalation of pharmaceuticals and environmental aerosols, equipping participants with essential insights in performing inhalation toxicological studies and interpreting current challenges and limitations of in vivo and in vitro inhalation models. Each topic will be presented by experts in the field and will include opportunities for the attendee to directly engage with the experts.
Chairs
Speakers
-
Ron Wolff
Inhalation studies: conduct, interpretation, relevance to humans -
Philip Kuehl
Practical delivery of aerosols in non-clinical systems -
Barbara Rothen-Rutishauser
Relevance of in vitro models for inhalation toxicology -
Fabian Blank
Animal models and the use of in vivo data for in vitro-in vivo extrapolation -
Jessica Oakes
Relating exposures between animals and humans through in silico modeling
9:00 - 12:00 Bioaerosol Sampling in High-Risk Settings to Understand Pandemic Respiratory Virus Transmission
This workshop will first include a seminar about a series of bioaerosol field sampling and clinical studies performed throughout Southeast Asia and in the U.S. prior to and during the initial years of the COVID-19 pandemic. Attendees will gain a big-picture perspective of bioaerosol research in the context of emerging infectious diseases and pandemic preparedness. Following the seminar, an optional tour of the Public Health Aerobiology Laboratory (PHAB Lab) at the UMD School of Public Health will be offered. During the tour, attendees will get the chance to view some of the eminent research equipment used to shape our collective understanding of airborne transmission and the course of current and future pandemics.
Chairs
Speakers
- Details coming soon
9:00 - 12:00 Aerosols Basics
This workshop provides a foundational overview of aerosol drug delivery. It covers the physical, anatomical, and physiological principles that influence aerosol deposition, as well as considerations for pediatric patients and innovations in device technologies. Designed for attendees new to the field or those looking to refresh their understanding, the session integrates expert-led presentations on in vitro and in vivo factors, device design, and future directions in aerosol medicine.
Chairs
Speakers
-
Anthony Hickey
Basics of Respiratory Drug Delivery I: Physical principles of aerosol drug deposition (in vitro) -
Hui-Ling Lim
Aerosol Devices: Comparison of functional principles and design elements -
Chris O'Callaghan
Basics of Respiratory Drug Delivery II: Anatomical and physiological principles affecting aerosol deposition in vivo -
Israel Amirav
Pediatric Considerations: Differential device selection and integration of device to patient -
Jim Fink
Innovation in Aerosols in Medicine – From smart nebulizers to critical care to vaccines
9:00 - 12:00 Modeling and Simulation
This workshop brings together experts in computational and experimental modeling to highlight cutting-edge approaches in simulating inhaled drug delivery. Topics span regulatory frameworks, model-informed drug development, whole-lung deposition, and the integration of in vitro and in silico methods to better predict clinical outcomes. Attendees will gain insight into the role of modeling in both early-stage research and regulatory approval processes.
Chairs
Speakers
-
Catherine A. Fromen
The Next Frontier in Inhalation Modeling: Developing experimental tools to capture disease-specific whole-lung deposition and cellular responses -
Gur J. Pal Singh
Modeling and simulation in approval of inhaled generics drugs: Success stories and possibilities -
Jan de Backer
Functional respiratory imaging: Toward quantitative medicine and in-silico trials -
Ross Walenga
Model integrated evidence and Model Master File for developing orally inhaled drug products -
William Ganley
The role of PBPK modelling in translating in vitro data to clinical outcomes: Product development and regulatory perspectives -
Andy Clark
Modeling preterm and newborn lung deposition
9:00 - 12:00 Propellant Transitions in pMDIs
Recent regulatory developments have prompted a shift in the types of propellants used in pressurized metered-dose inhalers (pMDIs), driving changes across the field. This session will provide updates on evolving regulatory guidance and highlight industry experiences related to the transition to next-generation propellants in pMDIs, including perspectives on formulation, manufacturing, clinical performance, and technical considerations.
Chairs
Speakers
-
Thomas O’Riordan
Welcome -
Svetlana Lyapustina
Global transition to new generation propellants – current context -
Ross Errington
The Transition to Next Generation Propellant Metered Dose Inhalers: A Technical Overview -
Enrico Zambelli
Formulation Strategies for Next Generation Propellants Transition in MDI: Ensuring a Smooth Transition for Multi-Drug Solution and Suspension Products -
Lucas Silva
Alternative in vitro bioequivalence approaches for the low GWP propellant transition -
Sheryl Johnson
Propellant manufacturer perspective: HFA-152a -
Rahul Parakhia
Propellant manufacturer perspective: HFO-1234ze -
Marco Laackmann
An Alternative Approach for Pressurized Metered Dose Inhalers Manufacturing to Facilitate Propellant Transition -
Bryan Newman
FDA perspective on MDI propellant transitions – from generic drug perspective -
Stacy Chin
FDA Perspective on MDI Propellant Transitions – from New Drug Perspective -
Omar Usmani
Transition to low carbon inhalers: A clinical perspective -
All the speakers, Partha Roy and Bing Li
Panel discussion
9:00 - 12:00 Intranasal Delivery of Aerosolized Vaccines
This workshop explores the development and delivery of aerosolized vaccines via the intranasal route, covering preclinical and clinical advances, device technologies, formulation strategies, modeling approaches, and funding opportunities. Attendees will gain a broad understanding of the scientific and translational challenges of intranasal vaccine programs from leading researchers and developers in the field.
Chairs
Speakers
-
Xiaoping Zhu
Overview of intranasal vaccines and how they work -
Phillip Kuehl
Preclinical study considerations -
Julie Suman
Vaccine device landscape -
Goncalo Farias
Formulation and manufacturing aspects -
Beth Laube
3D intranasal model casts and intranasal delivery of aerosolized vaccines -
P. Worth Longest
Computational fluid dynamic (CFD) modeling and intranasal delivery of aerosolized vaccines -
Laura Powell
Funding options for intranasal aerosolized vaccine research -
Elizabeth Norton
Adjuvanted intranasal Klebsiella vaccine -
Xiaoping Zhu
Transmucosis intranasal vaccine program -
Michael Egan
Castlevax's next generation mucosal vaccine platform
12:30 - 1:30 Lunch Break
Women in ISAM Lunch
ISAM has a strong tradition of supporting women scientists and has the networking base to support the next generation of women researchers. The ISAM Congress 2025 in Washington DC is organizing a Lunch Session to bring together researchers of all gender identities to network and engage in a dialogue with other ISAM members. An open discussion will touch on how to overcome challenges of female scientists at all stages of their independent research careers.
ISAM Congress Schedule Overview
Sunday 22 June 2025
1:30 - 1:45 Conference Opening by the Congress Presidents
1:45 - 13:00 Session 1: What's New in Lung Inflammation and NCFBE?
This session explores emerging perspectives on lung inflammation and non-cystic fibrosis bronchiectasis (NCFBE), ranging from molecular targets for chronic cough to nanotechnology-driven immunotherapies and clinical management strategies. Attendees will hear from experts across academic and clinical fields sharing novel insights and potential breakthroughs in treating chronic airway conditions.
Chairs
Speakers
-
Imran Satia
Updates in the lung biology of chronic cough – new targets on the horizon -
Michael Trautmann-Rodriguez
Modulational of Macrophage EVs in Pulmonary Inflammation -
Gregory Tino
A clinical perspective on bronchiectasis
3:00 - 4:00 Social Break in Exhibit Area with Chaired Poster Session
4:00 PM – 5:15 PM Session 2: New Developments in Treatment of Severe Lung Diseases
This session highlights advances in the treatment of severe pulmonary conditions, including pulmonary hypertension, pulmonary fibrosis, and interstitial lung diseases. Speakers will present both current standards of care and promising inhaled therapies in development, offering translational insights for improving outcomes in complex respiratory diseases.
Chairs
Speakers
-
Paul Hassoun
Pulmonary hypertension and current treatment modalities -
Craig Conoscenti
Pulmonary fibrosis and the promise of inhaled therapies -
Vlad Malinin
TPIP, an inhaled prostanoid prodrug in development for PAH and PH-ILD
5:15 - 7:00 Wine & Cheese Reception in Exhibit Area
Monday 23 June 2025
7:30 am Registration Opening
9:00 AM – 9:30 AM Morning Plenary: Myrna Dolovich
My Reflections from ISAM: Past, Present, Future – Imaging
9:30 - 10:30 Flash Talks: CMC/Regulatory and Formulations
10:30 - 11:15 Coffee break
11:15 AM – 12:30 PM Session 3: Formulation and Devices – Marrying Formulation and Device
The success of an inhalable therapeutic relies on the harmony between the formulation and the device employed for administration. The advancement of pulmonary and nasal delivery of novel drug modalities, such as peptides, proteins, and nucleic acids, imposes new challenges on drug product development. This session highlights recent advances in pulmonary and nasal formulations and devices, with a focus on enabling delivery for emerging therapeutic indications.
Chairs
Speakers
-
Fransesca Buttini
Lpb. plantarum inhalation powder for the restoration of the lung microbiota -
Irene Rossi
GLP-1: Exploring respiratory delivery through the development of a GLP-1 analogue dry powder -
Gaurav Sahay
Microfluidic platform for shearless aerosolization of lipid nanoparticles for mRNA inhalation -
Kimberly Shepherd
Dry powder intranasal vaccines: Manufacturability and formulation considerations in an emerging area -
Hugh D. Smyth
The future of patient-friendly high-payload inhaled therapies
12:30 - 1:45 Lunch Break
Young Investigators Lunch
ISAM aims to support young group leaders and scientists working in aerosol medicine. The ISAM Congress 2025 in Washington DC is organizing a Lunch Session to bring together researchers who are in the early stage of their career and to provide contact to and a dialogue with already established senior group leaders. An open discussion will touch on how to overcome challenges of young scientists, when starting their independent research. The event is Sponsored by Aerogen Pharma.
JAMPDD Lunch
1:45 PM – 3:00 PM Session 4: Best Oral Presentations
This session features outstanding graduate student research presentations selected from submitted abstracts. Each talk represents cutting-edge work in inhalation science, with finalists competing for the “Best Oral Presentation” award. A live audience vote will determine the winner based on the quality and impact of their research and presentation.
Chairs
Speakers
-
Mélina Guérin
Delivering nanomedicines via inhalation: a novel pathway to fight lung cancer -
Narges Mirdamadi
Prediction of total and regional deposition efficiency of surfactant delivery in the preterm neonate respiratory system -
Chen-En Chiang
Molecular mechanisms underlying the therapeutic effects of inhaled UC-MSC exosomes in pulmonary fibrosis -
Julia Berends
INBRIJA versus LEVODOPA CYCLOPS: An in vitro – in vivo comparison of two orally inhaled levodopa dry powder products -
Scott Tavernini
A step towards region-specific in vitro deposition and dissolution: Characterization of an alveolar filter designed for dissolution measurements -
Emma Sudduth
Tuning hydrogel nanoparticle surface chemistry to highlight age-based changes in innate immune cell response to inhaled therapeutics
3:00 - 4:00 Social Break in Exhibit Area with Chaired Poster Session
4:00 PM – 5:15 PM Session 5: Environmental Aerosols, Climate, and Respiratory Disease
This session will discuss the interplay between occupational/environmental aerosols and global warming, leading to exacerbation of respiratory diseases, including asthma, allergic rhinitis, and influenza.
Chairs
Speakers
-
Akua Asa-Awuku
Impact of climate change on environmental air quality -
David Edwards
Global warming exacerbates airway mucosal collapse to provoke cough and cough hypersensitivity -
Tom O’Riordan
Asthma: Climate change, environmental aerosols, and asthma control -
Chantal Darquenne
Evidence of lung abnormalities in asymptomatic vapers
5:15 - 7:00 Wine & Cheese Reception in Exhibit Area
Tuesday 24 June 2025
7:30 am Registration Opening
9:00 AM – 9:30 AM Morning Plenary: Igor Gonda
My Reflections from ISAM: Past, Present, Future – Inhaled Drug Delivery
9:30 - 10:30 Flash Talks: Env/Occup and Devices
10:30 - 11:15 Coffee break
11:15 AM – 12:30 PM Session 6: Preclinical Efficacy Models
This session offers an insight into the latest advancements in the field of efficacy testing for respiratory-administered drugs. It highlights established in vivo models, innovative in vitro and ex vivo approaches, and cutting-edge AI-powered in silico tools. Speakers will discuss advantages, limitations, and regulatory aspects of these complementary strategies in preclinical drug development.
Chairs
Speakers
-
Emily (Resseguie) Hackshaw
In vivo respiratory disease models -
Armin Braun
Human-relevant ex vivo models for preclinical testing of new inhalational therapies against lung diseases -
Michelle Chen
AI-approaches in preclinical inhalation drug development -
All Speakers
Panel discussion
12:30 - 1:45 Lunch Break
Networking Groups
1:45 - 3:00 ISAM General Assembly
3:00 - 4:00 Social Break in Exhibit Area with Chaired Poster Session
7:00 - 9:30 ISAM Gala Dinner and Awards
Wednesday 25 June 2025
7:30 am Registration Opening
9:00 AM – 9:30 AM Morning Plenary: Tonya Winders
Perspectives from patients on inhalation treatment – current challenges and their future needs
9:30 - 10:30 Flash Talks: Pediatrics/Cystic Fibrosis(CF) and Imaging
10:30 - 11:15 Coffee break
11:15 AM – 12:30 PM Session 7: AI/ML and In-Silico Applications in Aerosol Research
The use of machine learning, artificial intelligence, and in-silico modeling is growing rapidly in healthcare and life sciences. This session will provide examples of how these technologies are being applied to aerosol-related research and how their use fits within the evolving regulatory landscape.
Chairs
Speakers
-
Ross Walenga
Evaluating charcoal block pharmacokinetics as a surrogate for regional lung drug delivery in orally inhaled drug products -
Yu Feng
Artificial Intelligence (AI) empowered user-centered smart inhaler for targeted drug delivery to designated airway sites -
H. Joy Sharp
An overview of FDA’s AI rules and guidance in the combination products space -
All Speakers
Q & A
12:30 - 1:45 Lunch Break
Networking Groups
1:45 PM – 3:00 PM Session 8: Translation to the Clinic
This session explores the critical steps and challenges in translating preclinical research into clinical applications, with a focus on both inhaled and intranasally delivered drug products. Featuring three expert speakers delivering focused presentations, the session addresses scientific, regulatory, and financial hurdles encountered during the transition from lab to clinic. Key topics include optimizing delivery platforms for clinical use, navigating regulatory pathways, and securing funding to support development. The session concludes with a dynamic panel discussion highlighting perspectives from researchers, clinicians, and industry experts—designed for scientists, entrepreneurs, and innovators aiming to bring therapies to the market.
Chairs
Speakers
-
Thomas Hofmann
The Story of Clofazimine from proof of concept to clinical asset -
Melissa Calton
Nonclinical development of 4D-710, an aerosolized gene therapy for cystic fibrosis lung disease -
Bill Thelin
The development of novel mucus-targeted therapeutics… from academic labs to clinical asset -
All Speakers
Panel discussion